26 Jul Researchers Travel to Micronesia to Test New Product for Tooth Decay
Investigators from the University of Washington (UW) and the ITHS Regional Clinical Dental Research Center traveled to Micronesia in order to conduct a clinical trial for a new interventional treatment to combat dental decay in children. This phase II clinical trial will evaluate the efficacy in preventing the disease known as “early childhood caries” or ECC. Caries is another word for dental decay caused by bacteria on the tooth’s surface, and “early” implies children under age six. Dental caries is the single most common chronic childhood disease in the U.S. and across the world.
The initial testing phase for a new treatment is called a phase I clinical trial. In this case, the treatment is a new fluoride product and the phase I trial, approved by the Food and Drug Administration (FDA), took place at the ITHS Regional Clinical Dental Research Center (RCDRC). During phase I, the primary goal was to ascertain the safety of the methodology for applying the new fluoride varnish to children. At the RCDRC, the trial participants (children 36 to 60 months of age) had a small amount of the new fluoride varnish applied to several teeth and were watched very closely to see if any negative effects were displayed.
To further confirm the product’s safety, additional information was gathered through another phase I trial on adults conducted in the RCDRC and at the Translational Research Unit (TRU). In this trial, any of the applied varnish that was absorbed or swallowed was measured through blood and urine analysis to verify that the amounts were within safe limits and were metabolized and discarded within several hours. Once these trials were completed and no adverse effects were observed, the FDA approved the study team to begin a Phase II trial in order to determine the product’s effectiveness. The research team traveled to Micronesia in order to work with their target population: children with ECC.

Research staff and Dr. Tut (standing right) explain the trial procedures and goals before requesting consent for participation
The phase II trial is designed as a randomized and double-blind trial – meaning the team would test the efficacy of the new product by comparing its impact with the current standard of care. In this case, the current standard of care is fluoride varnish, and the new product is fluoride varnish with an added antimicrobial intended to help fight the bacteria which cause dental caries (tooth decay). The children participating in the study were randomly assigned to receive either the regular varnish, or the varnish plus antimicrobial. To call a study ‘double-blind’ means that neither the research team nor the children know what version of the fluoride varnish they receive so that the results are unaffected by perception. Afterwards, the research team can analyze the data collected without worrying that it has been influenced by participants’ opinions about whichever varnish they received. This type of comparison allows researchers to isolate the effects of the antimicrobial fluoride varnish in their conclusions.
Marilynn Rothen, Clinical Associate Professor at the School of Dentistry, and manager of the RCDRC was among the team of researchers who traveled to Pohnpei, Micronesia for study implementation. Rothen works closely with Peter Milgrom, DDS, professor of Oral Health Sciences at UW, and Ohnmar Tut, BDS, affiliate faculty, Oral Health Sciences and past-president of the Pacific Basin Dental Association. Dr. Tut is located in Australia and has worked frequently with this population in the U.S. Affiliated Pacific Islands and serves as the Principal Investigator on the project.
While communities in Pohnpei do have access to dental care, regular preventative care is not widely utilized. Furthermore, the municipal water supply is not enhanced with fluoride as it is in some other countries, and the remote location of the Federated States of Micronesia can limit the supply of fresh fruits and vegetables available for purchase while much processed food arrives by ship. All these factors contribute to the prevalence of dental caries in Pohnpeian children, and make Pohnpei an ideal community for conducting a trial on ECC. The goal for the team was to recruit at least 280 trial participants.
They opened wide like little alligators and let me count their teeth.
In order to simplify the trial process for participants and their families, the study team conducted all trial procedures at local schools, beginning with parental consent. Rothen and the team spent four weeks in Pohnpei to train local staff on application techniques, and begin trial implementation. All study procedures will be completed within two years, with the most intensive processes taking place during those initial four weeks.
- First, the parents of each potential participant must give their consent. During consent, the study team explained the trial procedures and goals to the families and answered any questions they had about the study. During their first two weeks in Micronesia, 299 parents gave consent for their children to participate in this research.
- During the second week, the team began initial exams. Exams must be conducted before treatment, at one year, and at two years to assess the impact of the trial product. Exams were performed for 285 children. The primary outcome of this study is to document the change in tooth decay through ‘before and after’ comparative exams.
- Application of the fluoride treatment began in week three. The children will continue receiving varnish every 3 months for two years.
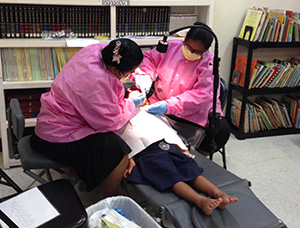
The exams and varnish application were conducted in classrooms using portable dental equipment. The children participated in the study from the comfort and familiarity of their school environment.
One important piece of data that needed to be collected was a quality assessment, “we wanted to find out the children’s reaction to the new fluoride varnish” says Rothen. The team needed to document this reaction in a measurable way for each of the study participants. The team worked to develop a culturally appropriate phrasing of this question, and translated it into Pohnpeian. The exact same phrasing was used for each child. To have a measurable answer, the child was then asked to point to one of five mood indicator images as a response to the question. The research staff was instructed to resist the urge for further explaining the question, or continuing to prompt the child for an answer. “What I found was as long as you are talking at the child, the child just sits there and will not point to something” said Rothen. Pausing is what gave the child permission to respond.
The research team will return to Pohnpei, Micronesia at least two more times for follow-up exams and continued application of the investigational fluoride varnish. The team hopes to use evidence collected through this trial to show that adding an antimicrobial to fluoride varnish increases one’s ability to prevent tooth decay and treat early childhood caries. Treating this common condition can help children achieve life-long oral health by ensuring that the permanent adult teeth, which begin to erupt at age 6, do not get contaminated by decay-causing bacteria.