10 Feb PENUT Trial Seeks to Improve Well Being of Premature Babies
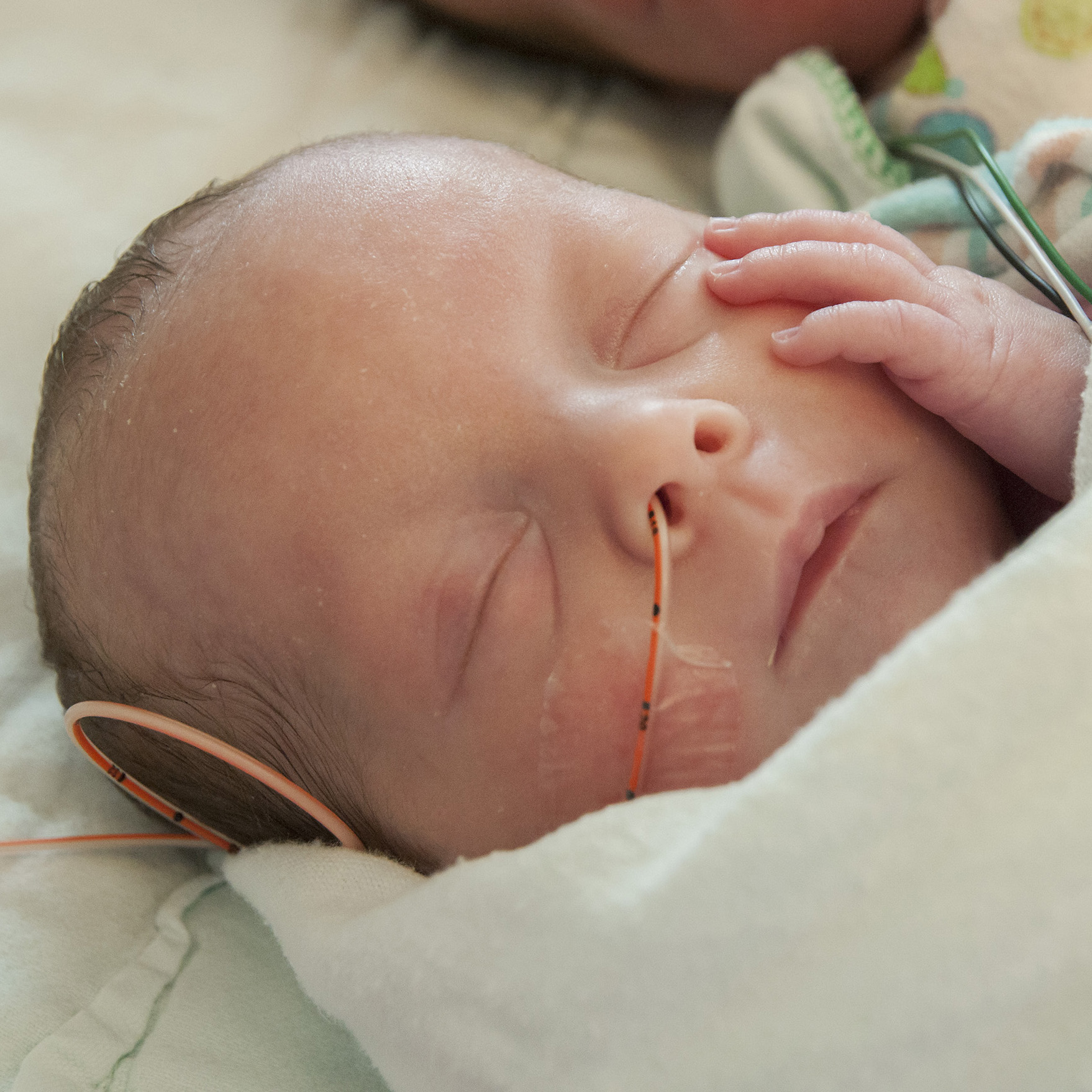
The PENUT (Preterm Epo Neuroprotection) trial is a new study being led by University of Washington researchers who are assessing whether erythropoietin (Epo) can provide neurological protection for infants who are born extremely prematurely. This study is seeking a treatment for the approximately 50,000 infants a year in the United States who are born at less than 28 weeks of gestation, which puts them at extreme risk of death, neurodevelopment impairment, and chronic health problems.
“The goal of this study is to see if Epo can help improve the health and neurologic outcomes of these babies,” said Dr. Sandra E. Juul, a Professor of Pediatrics at the University of Washington and the Principal Investigator for the Clinical Coordinating Center component of the study. “There is not a lot of comparative effectiveness research being done for premature infants, so this is a unique opportunity to help give them more of a fighting chance.”
This study will enroll a total of 940 extremely premature babies at 17 different trial centers across the nation. Half of the enrolled babies will receive injections of Epo at regular intervals until 33 weeks post-menstrual age, while the other half will receive a placebo during this period.
The study will track each baby’s development by phone every four to six months until they reach two years of age. At age two, the parents will complete a quality of life survey and the babies will be tested to assess their neurological development.
When developing the study, the PENUT study team wanted to avoid the risk of giving actual injections to the babies who are to receive the placebo. Their concern raised the challenge of how to still keep study personnel as blinded as possible, so they turned to the Institute of Translational Health Sciences (ITHS) for support.
ITHS is helping to address the team’s challenge by providing a research Registered Nurse (RN) to administer injections, or to fake the injections (i.e., for the placebo group), for all enrolled babies at the University of Washington study site. Only the ITHS RN and the pharmacist are aware of which babies at the site are actually receiving injections, which helps eliminates the potential for bias to insert itself into the study.
The Data Coordinating Center for the PENUT trial, which is led by Principal Investigator Dr. Patrick Heagerty, developed a custom portal for capturing and managing study data. The portal is based on REDCap, a web-based electronic data capture tool that ITHS offers for free to researchers in the five-state WWAMI region. The custom portal allows the roughly 150 users located around the country to randomize babies 24 hours a day and capture the data required to ensure the success and integrity of the trial.
“The tools and resources we are accessing through ITHS have been helpful in supporting the startup of the PENUT trial,” said Dr. Juul. “We look forward to their continued support during the rest of this study.”
Please visit the study site, penut-trial.org, to learn more about the PENUT trial, or visit www.iths.org to learn more about the services and tools that are available to researchers through ITHS.
Additional Coverage of PENUT Trial:
Seattle Children’s studies hormone that may save premature babies (KING 5, April 22, 2014)
Hormone may be vital in improving brain development in premature babies (On the Pulse, March 26, 2014)