CRISP: Clinical Research Intensive Summer Program
CRISP, the Clinical Research Intensive Summer Program, provides clinical investigators with hands-on experience and key clinical research skills to accelerate their career development. The course runs over 3 weeks in July and requires full-time commitment.
Application Period Open
Key Dates
- Now Accepting Applications
- Notification of Acceptance
Applicants will be contacted by January 13, 2025, or within two weeks after submission, whichever is later - Program Dates
July 7–25, 2025
The course will feature:
- Clinical Biostatistics and Epidemiology
- Choice of an elective: Quality Improvement or Clinical Trials
- Enrichment lectures, for example: best tips for writing career development grants, how to give a good talk and how to manage your time efficiently
- Opportunities to engage with experienced clinical investigators over lunches to discuss your individual projects
- Two optional minicourses: Introduction to R Programming and How to Mine the Electronic Medical Record
The course is intended for research fellows and early-career faculty who will have clinical investigation as a significant part of their careers. The course is designed for fellows who have completed their clinical training and are in their research time. Residents whose programs incorporate formal research years are also welcome to participate as are junior faculty conducting clinical research. All branches of medicine are welcome including medical, pediatric and surgical subspecialities, psychiatry, pathology, radiology etc. from any institution. Non-physician clinical investigators are also encouraged to apply.
Please note, all activities will be held in-person because small group projects, interactions with established clinical investigators, and bonding with other early stage clinical investigators are important parts of the experience.

CRISP Information Sessions
We’ll be hosting a few live information sessions for anyone interested in learning more about the program.
- CRISP Info Session 1 | Friday, January 24, 12–12:30pm PST
Register: https://washington.zoom.us/meeting/register/tJ0sdOmurDwqHtIsXNVQ_JaFnHiQyRhfurK_#/registration - CRISP Info Session 2 | Thursday, February 6, 12–12:30pm PST
Register: https://washington.zoom.us/meeting/register/tJMtceGvqjouGNY1mzn0ftHJ4EzUr5dJSP_6#/registration
Program Information
Prerequisites
What prerequisites are required?
Clinical training and familiarity with medical writing and publications are required because examples are drawn from the medical literature. No prior biostatistical or epidemiologic training is required. The electives do not require any prior experience.
Although it is not required for you to bring your own research projects, if you do, you will have an opportunity to discuss them with experienced clinical investigators and fellow students. Depending on the nature of the projects, you may also have an opportunity to develop them further in your classes.
Time Commitment
What is the time commitment?
The program will be held in-person on the Fred Hutch Cancer Center campus from July 7-25, 2025. Lectures are recorded and may be viewed after the class but there is no hybrid option. In-class discussion and engagement are viewed as critical components of the course, as are the interactions with classmates during class breaks, group projects, and social activities. During the course, students should be excused from most clinical responsibilities including clinics, inpatient and consult services, on-call etc. Beepers should be turned off during class. This is an intensive didactic experience with out-of-class assignments and group project meetings. Participants should plan on up to 4 hours of class a day and a similar out-of-class commitment throughout the 3-week period.
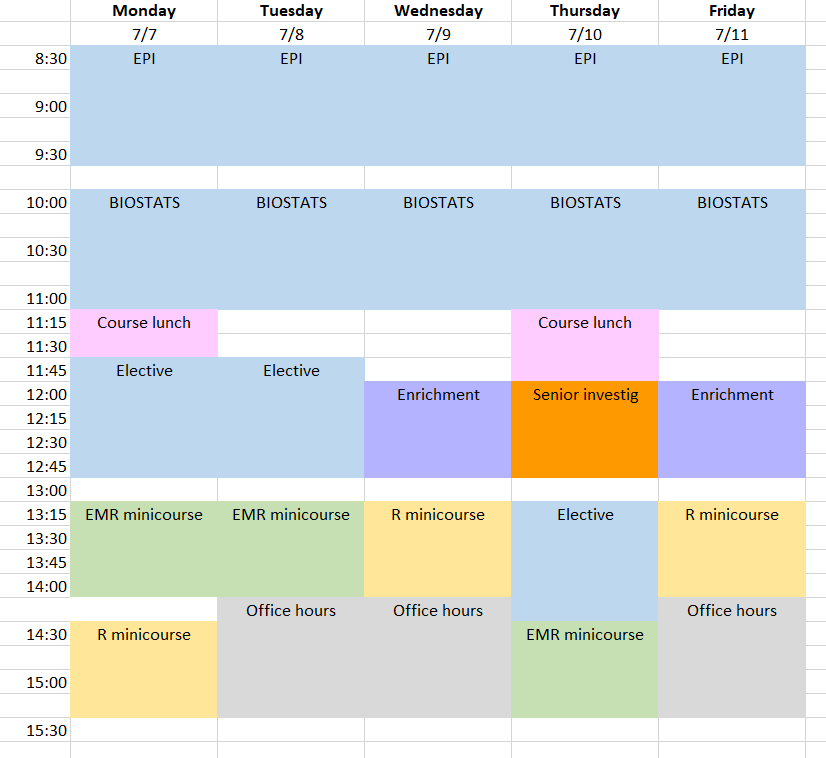
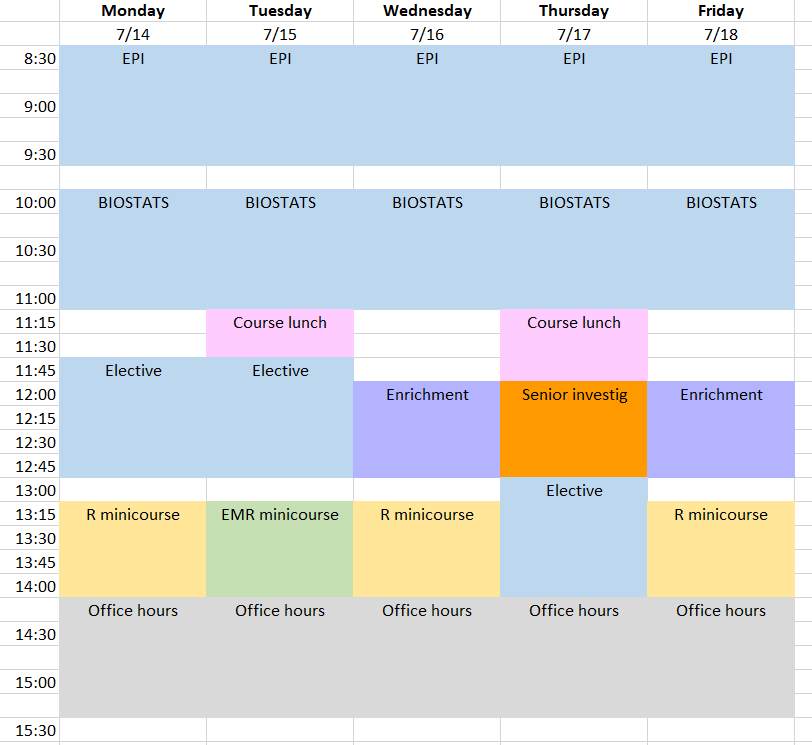
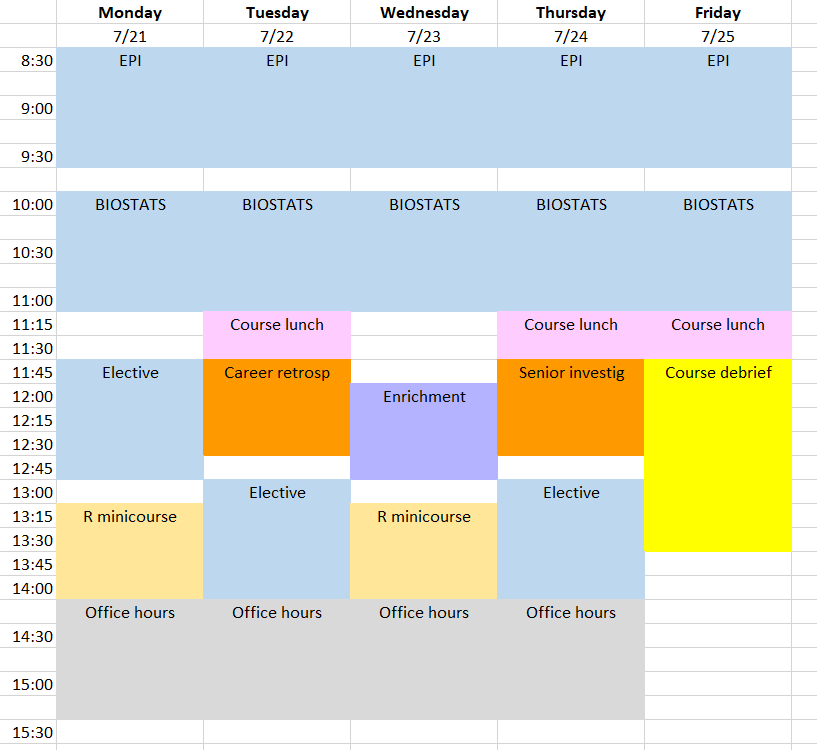
Class Schedule & Draft Daily Topics
Class Schedule
Download the 2025 CRISP class schedule here.
Morning Course Descriptions
NOTE: Course descriptions below are from CRISP 2024. Updated descriptions for 2025 are coming soon.
Participants will take both Epidemiology and Clinical Biostatistics in the morning.
Introduction to Epidemiology for Clinicians
This course will cover essential epidemiologic concepts, skills, and study designs with a focus on clinical research. Topics include measures of disease frequency and excess risk, overview of study designs, causal inference, screening, measurement error, misclassification, effect modification, and confounding. Students will engage with clinical literature throughout the course, building skills in understanding how and why to incorporate particular epidemiologic methods in clinical settings. Other out of class work includes problem sets that will challenge students to think creatively and critically about epidemiologic methods; computation will be minimal, but students will be pushed to apply concepts quickly and thoughtfully.
In the final week of the course, students will write a proposal describing a research question and related background literature, potential datasets or methods of gathering data, and an analysis plan. Students will receive feedback on their proposals from instructors and through peer review.
Clinical Biostatistics
The course will expose participants to some of the key statistical concepts relating to the analysis of data by using examples from the health sciences literature and example data. The topics will relate to questions such as: How many individuals do I need for my study? Do I have enough data to answer my research question? What is the best design to answer my research question? What criticisms of my study might I be able to anticipate?
For example, lectures will cover:
- basic study designs/approaches (experimental/observational and confirmatory/exploratory)
- basic analysis tools (descriptive stats and graphs including SD vs SE and dependent an independent variables, sample vs population)
- other specific topics: bias, confounding
- hypothesis testing, confidence intervals, power, multiple comparisons
- regression
- other topics depending on literature and data examples
Students will be given datasets so they can learn R programming to illustrate concepts introduced in class. No prior experience with R is required. Students familiar with R will have the opportunity to attempt more advanced problem sets.
Afternoon Course Descriptions
Participants will choose one of the options below for their afternoon course.
Improving Healthcare Quality and Measuring Outcomes with Epic Data
This course is designed for practicing clinicians and researchers with an interest in incorporating quality improvement (QI) methods in their work. This interactive and challenging course will prepare students to apply the Institute for Healthcare Improvement (IHI) Model for Improvement to the design and execution of a QI project. Students will also learn to use the robust self-service data tools within Epic that allow for measurement of both QI and research interventions. Topics of the session will include an introduction to widely used QI methods, understanding systems and quality drivers, QI and the IRB, developing quality measures, leading QI interventions, extracting data from Epic with Cogito self-service tools, statistical process control methods and software, and publishing QI interventions. Learners are welcome to bring a QI project to develop and kickstart during the course, or may work with hypothetical examples. Learners who complete the homework exercises will qualify for data access at UW Medicine as a Cogito Power User.
Clinical Trials
This short course is designed to introduce clinical investigators to the basics of designing, conducting and analyzing therapeutic clinical trials. Topics include key questions such developing an amenable research question, choosing the right study design, selecting primary and secondary endpoints, determining inclusion/exclusion criteria, and understanding what goes into the statistical section. Classes will address practical aspects of being a Principal Investigator, such as overall responsibilities, how to lead a team, how to set up a study database, pitfalls that could make a study uninterpretable, regulatory mistakes, budgeting and funding. Classes may include panel discussions on topics related to career development, including:
- How to get started as a local Co-investigator in an externally sponsored study
- How to make the transition to local PI for an externally sponsored study
- How to launch and conduct an investigator-initiated trial with Pharma support
- How to navigate an IND application and trial under FDA oversight
- How to make best use of monitors and coordinators
Out of class work will include readings, videos and working on the concept sheet or letter of intent for an interventional trial that you could pursue after the course.
Program Cost
What is the cost of the program?
The cost is $3,000 for the 3-week course, payable to Fred Hutch Cancer Center. It is hoped that tuition will be paid by others (Departments, Divisions, Programs or mentors) rather than by students. Costs cover instruction, course materials, welcome reception and final celebration, and some lunches.
Tuition is due for accepted applicants by May 30, 2025 to reserve your place in the course.
Application
What does the course application entail?
Applications will be accepted starting November 12, 2024, and students will be notified starting January 13, 2025. Click here to download a PDF preview of the application. Applications sent after January 13th will be considered on a space-available basis. Applicants should also provide the following:
- A C.V.
- A brief personal statement of interest (fewer than 100 words, about 500 characters including spaces)
When will I know if I am selected to participate?
We will review your application and contact you by January 13, 2025 or within the next 2 weeks, whichever is later. Please notify us if you do not hear from us within that time frame. All components of an application are required before it is reviewed. If necessary, a waitlist will be kept in case space opens. The enrollment maximum is 60 because of classroom size.
Other Clinical Research Training Opportunities
What other clinical research training opportunities are available?
The table below lists some options and describes how the Clinical Research Intensive Summer Program (CRISP) differs.
| S | Structure | How CRISP is different |
|---|---|---|
| MS or MPH (EPI and HS) | 2 years (MS $20k/year; MPH $24k/year) Online MPH, ~$60k total | Not degree-granting Intensive, focused, applied |
| The Summer Institutes (Statistics) | Minicourses, 1-3 full days, many intended for statisticians (Dates tbd) | CRISP does not require prior stats training |
| Clinical Research Methods Course | 10 weeks, 90 minutes/week, EPI (Winter) For more info, contact Dr. Kestenbaum (brk@uw.edu) | More intensive and applied |
| ITHS/UW SOM/UW SPH Grant Writing Program | Grant writing | Does not involve grant writing, but allows for the development of potentially fundable research questions and analysis plans For more info, contact Drs. Kessler (kesslerl@uw.edu) or Fitzpatrick (fitzpal@uw.edu) |
| ITHS training opportunities | Webinars, short courses | More intensive, applied |
| QI Scholars Program | 1-year advanced training in QI research | More intensive, does not require a project to enroll |
After the Course
Do I get any academic credit or a certificate?
No. This course is not affiliated with the UW or School of Public Health and no academic credit will be given toward a degree. People who finish the course will get a Certificate of Completion.
What will I be asked to do after the course is finished?
Participants also agree to complete a feedback survey at the end of the course to provide critical input to improve the course. We are also interested in measuring the impact of the course on participants and their careers. Students will be asked to let us to contact them for 10 years for the purpose of ascertaining career accomplishments and will provide intermittent CVs or biosketches.
What metrics will be tracked?
We will ask you to provide career data because we expect that participation in this course will result in:
- Better understanding of applied research methods, measured by perceived self-efficacy in conducting your research projects
- Greater and more impactful long-term research productivity, measured by publications, grants and academic faculty positions and promotions
- A desire for more formal training in clinical research methods, measured by the number of students who pursue additional classwork, or MPH or MS degrees
Faculty Biographies
Faculty
Susanne May
 Dr. Susanne May is Professor of Biostatistics at the University of Washington (UW) and Director of the UW Clinical Trials Center. She has close to 30 years of experience in providing statistical support for observational studies and clinical trials and/or leading major data and clinical coordinating centers. She has taught a variety of biostatistical classes primarily to non-statisticians. She is a co-author on two biostatistical text books which are both known for their ability to convey complex statistical methodology to non-statistical audiences
Dr. Susanne May is Professor of Biostatistics at the University of Washington (UW) and Director of the UW Clinical Trials Center. She has close to 30 years of experience in providing statistical support for observational studies and clinical trials and/or leading major data and clinical coordinating centers. She has taught a variety of biostatistical classes primarily to non-statisticians. She is a co-author on two biostatistical text books which are both known for their ability to convey complex statistical methodology to non-statistical audiences
Jessie Seiler
 Dr. Jessie Seiler (jseiler@uw.edu) is a teaching fellow at the University of Washington School of Public Health. She teaches epidemiologic methods and courses related to structural racism and the social and political determinants of health. Her research focuses on race-based bullying and violence, using both quantitative and qualitative methods, as well as social network analysis tools. In her time at UW, she has been deeply involved with pedagogy design and curriculum planning in the Department of Epidemiology. Dr. Seiler earned her MPH from Emory University’s Rollins School of Public Health and her PhD in Epidemiology from UW’s School of Public Health.
Dr. Jessie Seiler (jseiler@uw.edu) is a teaching fellow at the University of Washington School of Public Health. She teaches epidemiologic methods and courses related to structural racism and the social and political determinants of health. Her research focuses on race-based bullying and violence, using both quantitative and qualitative methods, as well as social network analysis tools. In her time at UW, she has been deeply involved with pedagogy design and curriculum planning in the Department of Epidemiology. Dr. Seiler earned her MPH from Emory University’s Rollins School of Public Health and her PhD in Epidemiology from UW’s School of Public Health.
Andrew White
 Dr. Andrew White is the director of education at the UW Center for Scholarship in Patient Care Quality and Safety, a Professor of Medicine, and a practicing hospitalist at UWMC. He is also a board-certified Clinical Informaticist and the UW Assistant Chief Medical Information Officer for clinical decision support. His research has focused on communicating with patients after harm, medical reasoning, and using IT to improve safety and value. Dr. White will be joined by a team of colleagues at UW and Seattle Childrens who regularly co-teach classes on Quality Improvement.
Dr. Andrew White is the director of education at the UW Center for Scholarship in Patient Care Quality and Safety, a Professor of Medicine, and a practicing hospitalist at UWMC. He is also a board-certified Clinical Informaticist and the UW Assistant Chief Medical Information Officer for clinical decision support. His research has focused on communicating with patients after harm, medical reasoning, and using IT to improve safety and value. Dr. White will be joined by a team of colleagues at UW and Seattle Childrens who regularly co-teach classes on Quality Improvement.
Paul Martin
 Dr. Paul Martin is a Professor Emeritus at Fred Hutch and the University of Washington. He has more than 45 years of clinical and research experience in hematopoietic cell transplantation with an emphasis on clinical trials and retrospective studies related to acute and chronic graft-versus-host disease. He served as Medical Director of Clinical Research Support (i.e., the clinical trials office) at Fred Hutch from 2011 to 2017. Dr. Martin is the course director for CRISP’s Clinical Trials elective.
Dr. Paul Martin is a Professor Emeritus at Fred Hutch and the University of Washington. He has more than 45 years of clinical and research experience in hematopoietic cell transplantation with an emphasis on clinical trials and retrospective studies related to acute and chronic graft-versus-host disease. He served as Medical Director of Clinical Research Support (i.e., the clinical trials office) at Fred Hutch from 2011 to 2017. Dr. Martin is the course director for CRISP’s Clinical Trials elective.
Course Organizer
Stephanie Lee
 Dr. Stephanie Lee (sjlee@fredhutch.org) is the Fred Hutch Co-PI for the Institute of Translational Health Sciences (ITHS) and a Professor at Fred Hutch and the University of Washington. She has been an NIH-funded investigator for 25 years and leads the Chronic Graft-vs.-Host Disease (GVHD) Consortium, a multi-center group dedicated to observational and therapeutic studies for this complication of blood and marrow transplantation. She earned her M.P.H. from the Harvard School of Public Health in 1996 after participating in a similar summer program during her hematology-oncology fellowship.
Dr. Stephanie Lee (sjlee@fredhutch.org) is the Fred Hutch Co-PI for the Institute of Translational Health Sciences (ITHS) and a Professor at Fred Hutch and the University of Washington. She has been an NIH-funded investigator for 25 years and leads the Chronic Graft-vs.-Host Disease (GVHD) Consortium, a multi-center group dedicated to observational and therapeutic studies for this complication of blood and marrow transplantation. She earned her M.P.H. from the Harvard School of Public Health in 1996 after participating in a similar summer program during her hematology-oncology fellowship.
Contact
For administrative questions, please contact:
Testimonials
Everyone was outstanding and I feel so fortunate to have had this amazing teaching team.
I thought the Biostats and Epi instructors were fantastic.
I loved all of the content. I agree with having more dedicated time for R and bringing in slicer dicer for those who did the outcomes elective (which I loved).
Working in small groups to design PROs was very helpful. This hands-on time is high yield.
The formal approach to QI projects was really eye opening. I enjoyed learning some of the more formal methods approaches (SPC, driver diagrams, etc.). Working through the process with individual projects was helpful to grasp the topics.
[Meetings with senior investigators were] Extremely helpful. I was able to utilize their knowledge to understand my research better. Great insight into ways to look further into my data.
These [enrichment lectures] were outstanding and the material was not only relevant to career development but demystified so many things.
They [enrichment lectures] covered a wide range of topics at an appropriate level for fellows/junior faculty and were very engaging and helpful. They gave concrete, actionable advice.
Great that this class is multidisciplinary because it allowed a lot of sharing between departments that does not really happen.
I really enjoyed getting to know the other investigators in this cohort.