
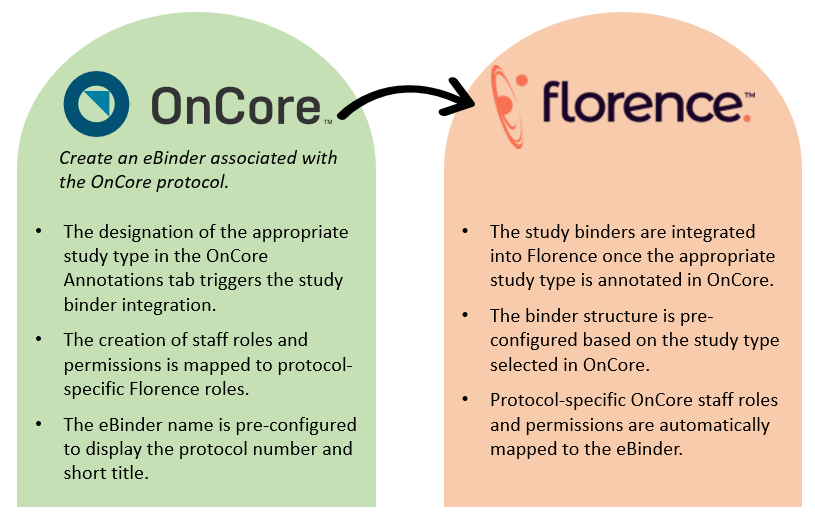
The UW Medicine Clinical Trials Office (CTO) implemented the Florence eBinders system for all UW clinical research studies, except for hematology and oncology, starting on March 1st, 2024. This system includes an integration with OnCore CTMS that enables the automated setup of study binders and user permissions in Florence based on the information recorded in OnCore.
The creation of eBinders is facilitated through system integration, which is determined by the OnCore Management Group and the designation of the appropriate binder type. OnCore staff roles are mapped to protocol-specific Florence eBinder roles to ensure seamless integration of permissions.
Florence remains the source of truth for all regulatory documents. Although the use of the Florence system is encouraged due to its efficiency and compliance, it is not mandatory. Each study team can decide when to begin using the system.
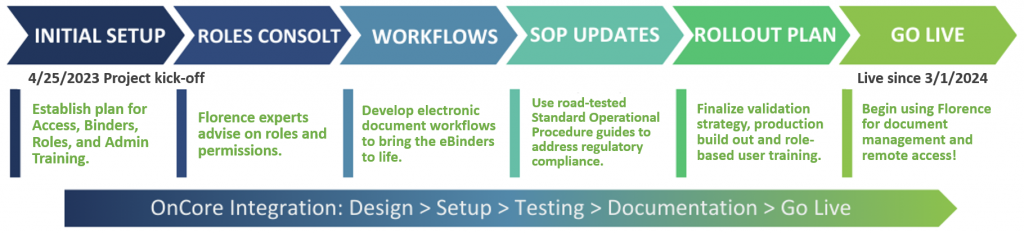
Project Goals & Timeline
FILE MANAGEMENT
- Eliminate paper binders. Manage and store regulatory, source, business, and other clinical research documentation securely in a 21 CFR Part 11 compliant system.
- Easily assign user roles and set up study binders with a consistent filing structure based on preset templates.
- Efficiently produce, sign, and manage electronic Delegation of Authority and other logs.
- Allow the system to keep track of and remind users about document expiration dates, renewals, and other due dates.
- Locally manage access to files and functional permissions, keeping user data safe and secure.
TIME MANAGEMENT
- Shifting to a web-based platform allows Investigators (PIs) and study teams to manage binders, review, and electronically sign documents securely from anywhere, using any device.
- No more waiting to corner PIs in clinic hallways with papers to sign that they have no time to review. PIs can review and sign documents on their own schedule.
- The electronic signature process is easy and quick.
- Documents are always updated in real-time and across all study binders where applicable.
REMOTE MONITORING
- Efficiently enable compliant, secure, and permission-controlled remote monitor access.
- Full audit trails assist with monitoring and verification of changes.
TRAINING AND TECHNICAL SUPPORT RESOURCES
- Robust online training library and resources.
- Focused training for PIs makes onboarding quick and easy, approximately 15 minutes.
FAQ (Frequently Asked Questions)
GENERAL QUESTIONS
What is the Florence eBinder system?
Florence is a 21 CFR Part 11 compliant electronic study binder that provides remote, digital access to study files from start-up to close-out. It enables the creation, editing, distribution, signing, and review of study files electronically. Please note that completion of training is required to access the Florence eBinder system.
Is the UW Florence eBinder system available for my study?
The UW Florence eBinders system is available for all UW clinical research studies, except for hematology and oncology.
Am I required to use the Florence eBinder system?
Although the use of the UW Florence system is encouraged due to its efficiency and compliance, it is not mandatory. Each study team can decide when to begin using the system.
What does the OnCore Integration entail?
The integration with OnCore CTMS enables the automated setup of study binders and user permissions in Florence based on the information recorded in OnCore.
The creation of eBinders is facilitated through system integration, which is determined by the OnCore Management Group and the designation of the appropriate binder type. OnCore staff roles are mapped to protocol-specific Florence eBinder roles to ensure seamless integration of permissions.
Can I still use the Florence eBinder system if my study is already in OnCore? What about studies that are not in OnCore?
Studies already in OnCore or those that do not require an OnCore presence will be set up directly in Florence upon request. Please send the request to uwctocomms@uw.edu.
Who do I contact if I have additional questions about UW Florence and the OnCore Integration?
For questions regarding the UW Florence eBinder system, including access requests and the OnCore integration, please contact the CTO at uwctocomms@uw.edu.
SYSTEM ACCESS AND USER TRAINING
I’m interested in using the UW Florence eBinder system for my studies. How do I request access?
When your study team is ready to implement Florence, CTO will grant access to the system and provide product demonstrations and training resources. Once access is granted, you will be able to log into the system using your UW NetID.
To obtain access to the Florence training environment, please email the CTO at uwctocomms@uw.edu.
What can I expect from the user training?
User training completion is required to gain access to the UW Florence eBinder system before setting up any study binders.
Training is tailored to specific roles, e.g., PI, Monitor, Study Coordinator, etc., and varies in time depending on the role. While PI training takes approximately 15 minutes, coordinator training may take a couple of hours.
CTO will provide a list of all Florence training requirements for each specific role and will verify and track training completion.
Pricing
How much does it cost to use the Florence eBinder system?
Industry-initiated studies:
- Each new industry-initiated study will be assessed a one-time fee of $2,175 (not inclusive of F&A) at the time of study binder setup.
- When the CTO made the initial announcement in 2023, study teams were asked to include this fee in study budgets for negotiation with industry sponsors. Please plan to negotiate this fee with your sponsors.
- Each industry-initiated study with an intake date on or after January 25, 2023 (the initial effective date of the Florence-UW agreement) will be assessed this fee. However, the fee may be covered internally by the CTO on a case-by-case basis for studies that did not negotiate the fee and have a finalized budget.
- If you need assistance negotiating this and other fees, the CTO will provide budget negotiation services at no cost to investigators (the CTO negotiates its own fee directly with the sponsor). To request budget negotiation services, please contact us at uwctocomms@uw.edu.
Investigator-initiated studies:
All investigator-initiated studies can access the full features of the UW Florence eBinder system at no cost to investigators.


