
An Ancillary Review refers to an external service or committee that requires approval before a research study can Open to Accrual (OTA). Some examples include the FHCC Cell Processing Facility, FHCC Institutional Biosafety Committee, UW OncoRad, and UW Total Body Irradiation.
The objective of the Ancillary Review Migration is to ensure approval is in place for required reviews before opening a research study to accrual. By migrating these types of ancillary reviews into the OnCore Clinical Trial Management System (CTMS), the CTMS team can manage required reviews and monitor study statuses in a centralized system.
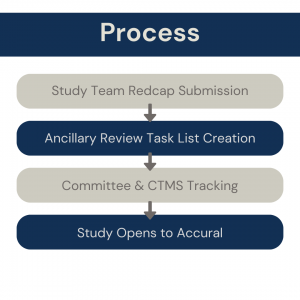
Based on the documents/information the study team submits in the REDCap Intake Form, an Ancillary Review task list will be created in OnCore CTMS. The service/committee will track the completion of required reviews in this task list. OTA is held until all required reviews are completed.
Project Goals
RedCap Reformat
REDCap will be reformatted so that Study Teams will submit documents/ information in the REDCap Intake Form.
A question will be added to the REDCap Intake form to:
1. Determine if your Service/Committee Review will be required.
2. Ensure that the information you need is collected in the REDCap form.
3. Create a notification for the Service/Committee Reviews that will be sent with pertinent information when the applicable question is answered affirmatively.Notifications will then be sent to the Service/Committee if required.
Task List
The Service/Committee will track completion of required reviews in an OnCore Task List.
This Task List is created by the CTMS team based off the study team’s responses on the REDCap intake form.
Open to Accrual
Open to Accrual (OTA) will be held until all required reviews are completed.
Having this centralized tracking system creates greater visibility and efficiency for the OTA process.
Document Submissions
Submission of documents/information related to amendment during startup and post-startup amendments will follow the same process (excluding Open to Accrual).
Project Workflow & Timeline
Coming Soon!


