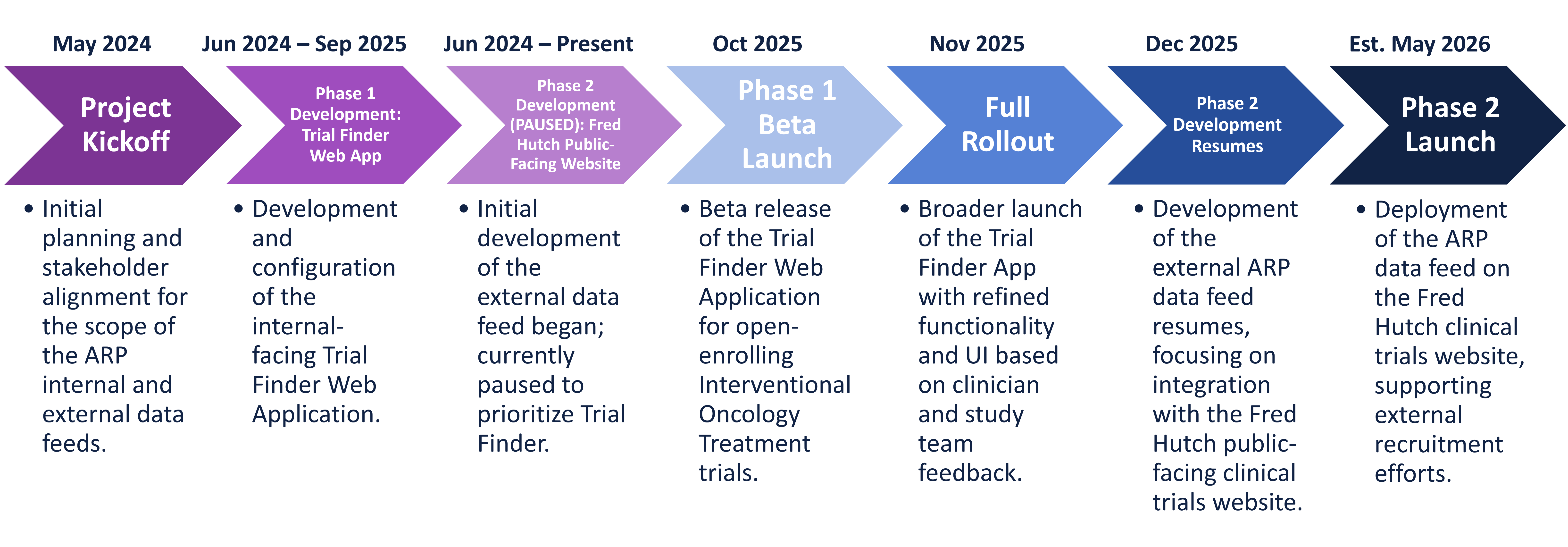
Overview:
The Advarra Research Portal (ARP) is a data feed from the OnCore Clinical Trial Management System (CTMS). This feed includes protocol information that Fred Hutch (FH) and the University of Washington (UW) can use to make trial details available for recruitment through both external clinical trial websites and an internal-facing web application.
Phase 1: Internal Trial Finder Web Application
- Development of a login-based, responsive-designed Trial Finder Web Application (link to dedicated project page)
- Goal: Enable clinicians and care teams to access trial-specific data in a user-friendly format to support review and discussion with prospective participants.
- The initial release will include Oncology-related studies that:
- Have a Treatment component
- Are categorized under the Interventional Data Table 4 Report Type.
- The configuration process will be designed and implemented from OnCore CTMS.
Phase 2: External Data Feed
- Development of a data feed to populate trial information on the Fred Hutch and UW public-facing clinical trials websites.
- Goal: Improve the quality, scope, and timeliness of data available for external recruitment efforts.