
This list will be continuously updated as feedback is received. Please check back periodically for updates.
Billing
How quickly will subject status information be available in Epic?
OnCore subject statuses flow to Epic near-time and will be available shortly after entry into OnCore. To ensure billing compliance per policies UW Medicine and Fred Hutch, subject status entries should be updated in OnCore within 24-hours of the actual event.
IMPORTANT: Do NOT register or update subject status in Epic.
Data Validation
What is the OnCore definition/functionality of On Study?
On Study is defined as the date the study subject has been registered to the study after having completed the eligibility step and has received a subject ID from the sponsor. The On Study date is the required date in OnCore for accrual credit. It is not related to billing. In the CTMS workflow, subjects will be marked as Billing Active in Epic as soon as they have consented to the study and have a consented date recorded in OnCore.
How do you differentiate between On Follow Up Date and Off Treatment Date?
The Off Treatment Date is the date the study subject stops the treatment phase of the protocol. They are not expected to receive any further interventions or enroll in other Treatment Arms. After an Off Treatment Date is added, the subject may move to Follow Up or Off Study.
IMPORTANT: For studies tracking subject visits in a study calendar, after the Off Treatment Date and/or On Follow-Up Date is added, you may not be able to complete visits from On Treatment phase of the protocol.
Document Upload & Access in OnCore CTMS
What Document Type name should I select for my document?
Document Type names should match the type document you are uploading.
IMPORTANT: Document Type names should be unique to each document and remain consistent throughout the life of the study. For example, if there is more than one consent form, assign “Main Consent” to the first consent form and “Main Consent A” to the second. Whenever an updated version is uploaded or re-entered during annual reviews, keep the same Document Type name. This is important for version control in Document Search and the CRA Console and for re-consent workflows to function.
Who will access documents from OnCore CTMS?
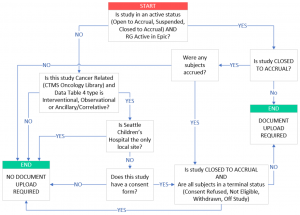
Which protocols are required to upload documents to OnCore CTMS?
- Epic status can be viewed in the PC Console > Status tab
- a) Has consent form documents
- b) Has a Data Table 4 Type of Interventional, Observational, or Ancillary/Correlative
Who do I contact if a document in OnCore is missing or the version is not correct?
What documents are available in OnCore’s Document Search?
The following study documents must be maintained by the study team in OnCore:
- Study Protocol
- Protocol Memo’s and Clarification Letters
- All Consent-type documents
- Investigator/Drug Brochure
- Drug Package Insert, Drug Booklet, or Drug Information Sheet
- All study manuals, including Pharmacy, Apheresis, Imaging, Laboratory, and Device Manuals.
The BMT Standard Practice Manual, BMT Standard Treatment Plans and CCO Book are still available in CORE.
What documents will be required to be uploaded?
1. Study teams will upload IRB approved documents to the PC Console > Reviews > IRB > IRB Review Update tab, within the IRB review the document was approved. IRB documents must be uploaded under the Initial Review, Continuing Reviews, and Modifications. Required documents include:
-
- Protocol
- Consent(s) and Assent(s)
- Investigator/Drug Brochure
- Protocol Memos and Clarification Letters
- Additional documents as desired
2. Study teams will upload study manuals to the PC Console > Documents/Info > Attachments/Links tab prior to open to accrual and whenever a new version is released. Required study manuals include:
-
- Lab Manual
- Imaging Manual
- Pharmacy Manual
- Product Manual
- Apheresis Manual
- Other Manual
How can we search OnCore CTMS for documents?
Using the Document Search functionality in OnCore CTMS (Menu > Protocols > Document Search).
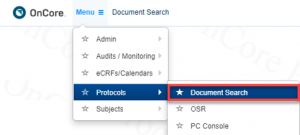
You can search for documents with any of the following protocol identifiers:
- Protocol Number (RG#)
- IRB Number
- Pharmacy Number
- Sponsor Number
- IND Number
- IDE Number
- Investigator
- Keywords: Any word found in the title, short title or objectives
Is it possible to print the consent footer and date of printing from OnCore?
The functionality to print a consent footer will be available in OnCore CTMS when document access goes live. See the Document Search & Print Job Aid.
General CTMS
What studies are required to be in OnCore?
All UW/Fred Hutch Epic-active protocols and all oncology-related and Fred Hutch non-oncology human subjects research are required to have an OnCore protocol record.
- Epic active protocols include all protocols with clinical activities, billing and/or orders, or protocols that need to release subjects to monitors in Epic.
- Oncology and FH non-oncology human subjects research include interventional, ancillary/correlative and observational protocols, as well as data collection (repository) protocols.
Study Start-Up
How do I submit a new protocol? How do I submit a study amendment?
What studies are required to be in OnCore?
All UW/Fred Hutch Epic-active protocols and all oncology-related and Fred Hutch non-oncology human subjects research are required to have an OnCore protocol record.
- Epic active protocols include all protocols with clinical activities, billing and/or orders, or protocols that need to release subjects to monitors in Epic.
- Oncology and FH non-oncology human subjects research include interventional, ancillary/correlative and observational protocols, as well as data collection (repository) protocols.
UW Workday - OnCore Integration, July 2023
What studies are in scope for the UW Workday – OnCore Integration?
UW Oncology and Non-Oncology studies are in scope for this integration if UW is the holder of funds and studies have calendars and financials built out in OnCore. Study teams should begin checking in visits via the study calendar, generating all applicable invoices, tracking receipts and reconciling payments as soon as the first Study Calendar is ‘Released.’
What training is available to prepare for the UW Workday – OnCore Integration?
During the month of June, CTMS Program Office and UW Finance Transformation team will be hosting joint weekly training sessions for our OnCore community (Date/Time TBD). Training documents and videos will be available on the CTMS Resource Main Page. Post-go-live, CTMS Program Office will be hosting daily office hours to provide support.
Yes, MyFD will be replaced by Workday. Study teams may look up sponsor payment status on Workday or via the Unclaimed Deposits report on the UW Cash Receivables website. The GCA Cash Team will also proactively reach out to study teams for unclaimed payments.
Where can I find the slides and recordings of the training sessions hosted during the month of June?
Slides and recordings of the training sessions are available on the CTMS Resource Main Page. (Log in required)
Slides: Navigate to CTMS Documents, then scroll down to the OnCore CTMS Webinar Slides section to locate slides.
Recordings: Navigate to CTMS Videos, then look for the recordings under CTMS Webinars.
Who do I contact if I have additional questions about the UW Workday – OnCore Integration?
Please contact CTMS@fredhutch.org.
Will study staff be responsible for updating the budget number to the Grant Worktag in OnCore?
For existing protocols, the CTMS team will populate the Grant Worktag before the integration goes live. After go-live, the study staff will be responsible for updating this in the Internal Account Number field in the PC Console Management tab if there are changes to their Grant Worktag. If a Grant Worktag has not been assigned to your study at intake, you can use other applicable budget numbers or Worktags that your team is using for startup costs, but you must return to this field to update before RG activation and OTA to ensure you have the correct Grant Worktag to enable the sponsor invoicing functionality.
Will this Grant Worktag be used for postal services?
Study teams may use the Grant Worktag to invoice for postal services if it is considered a study-related activity and will be billed to the sponsor. Below is the UW School of Medicine (SOM) guideline on postal service charges:
With Finance Transformation now less than 6 weeks away, Creative Communications (C2) has implemented a solution for postage barcodes that can charge to a Work Tag or a set of Work Tags. Due to the complexity of Work Tags, the equipment used by UW Mailing Services will not be able to read and report data in postage barcodes if we embed Work Tags in the barcode. Because of this, we have established the Postage Account program to acquire billing information for postage for all of UW outgoing mail.
If you currently know what Work Tag or set of Work Tags you will want to have postage charged to, please use this link to access the Postage Account form to establish your account.
Postage Accounts & Postage Account Numbers (PANs) | Creative Communications (uw.edu)
In June, we will start offering the option of ordering envelopes, business reply products, and postage barcode stickers with a postage barcode that has your Postage Account Number embedded in it, instead of your budget number.
We recommend that if you expect to do any mailings early in July that you take advantage of the opportunity to preorder the envelopes, business reply products, and/or barcode stickers that you will need. We do ask that you not use any of these products with Postage Account Number barcodes until after July 1.
As a reminder, we recommend that you limit the quantity of envelopes you order to use until July 1. Leftover envelopes with budget number postage barcodes are essentially useless unless you update them by hand by placing a Postage Account barcode sticker over the budget number postage barcode.
Study teams should contact the Grant and Contract Accounting (GCA) Cash Team via ccats@uw.edu or gcacash@uw.edu for any questions regarding sponsor payments (including automatic EDC payments) or if correction is needed for already applied funds.
The integration will catch the error and Workday will not accept these invoices. This will show up in the Summary Report the CTMS team will be monitoring. In general, Workday will not accept invoices that have:
- Invalid Grant Worktag (e.g., GR991111)
- Incorrect Grant Worktag format (e.g., 23-4567)
- Missing Grant Worktag information in the Internal Account Number field in the PC Console Management tab in OnCore
- Incorrect or missing sponsor code in OnCore
- Missing Sponsor Protocol Number field in the PC Console Sponsor tab in OnCore
What will happen if the study team pre-populates the Other Invoice Number field?
The integration will automatically overwrite and replace any existing values in the Other Invoice Number field with the Workday invoice number when the invoice is accepted. To prevent loss of information, please use the Invoice Comments field for any invoice or budget numbers used for internal tracking.
How are EDC Automatic Payments going to be handled for this integration?
For payments that are sent to GCA:
Once payment is delivered to GCA, it will be recorded in Workday and held on account. Workday uses an algorithm to match the dollar amounts between sponsor payments and invoices. GCA Cash Team also goes through their process to proactively look for unclaimed payments and will reach out to the study teams directly to verify whether a sponsor payment belongs to them. Alternatively, study teams can track the payment status via the Unclaimed Deposits report on the UW Cash Receivables website. Study teams may create an invoice proactively if they are able to confirm the line items that would be accounted for in the received funds. Alternatively, study teams can retroactively create an invoice in OnCore once they are notified of the received sponsor payment. When Workday accepts the invoice, GCA Cash Team will match and apply the sponsor payment held on account. Once the payment application process is completed, Workday will return a payment file to OnCore with payment information for reconciliation.
For payments that are sent to study teams:
Study teams can receive sponsor payments but they cannot deposit or cash them, regardless of the payment type (e.g.,checks or ACH). As such, study teams must send payments to GCA. The received funds will be recorded in Workday and held on account. Study teams can concurrently create an invoice in OnCore with the line items accounted for in the received funds. Once Workday accepts the invoice, GCA Cash Team will match and apply the sponsor payment held on account. When the payment application process is completed, Workday will return a payment file to OnCore with payment information for reconciliation.
If a payment is found to be overpaid or underpaid before it’s sent to GCA, study teams should work with the sponsor to correct it. After the payment is sent to GCA, a correction request can be submitted to the GCA Cash Team at ccats@uw.edu or gcacash@uw.edu.
Workday goes through a series of matching rules which depend on the data provided along with the payment. If there is not a clear 1:1 match, as would be in the case of one payment applying to multiple invoices, Workday would offer suggestions for which invoices the payment might need to be applied to and it would be up to the central cash team to select the appropriate invoices to complete the payment application process.
The GCA Cash Team goes through their process to proactively look for unclaimed payments and will reach out to study teams directly to verify whether a sponsor payment belongs to them. Alternatively, study teams may track the payment status via the Unclaimed Deposits report on the UW Cash Receivables website. Please note that payment information will not return to OnCore until a sponsor payment has been applied to an invoice. As such, it is up to the study teams to locate their own unclaimed payments from the Unclaimed Deposits report or reach out to GCA Cash Team at ccats@uw.edu or gcacash@uw.edu for payment status updates.
Study teams may verify the accuracy of payments with sponsors and against completed events/activities in OnCore before sending it to GCA. Once the payments are deemed accurate, study teams must send them to GCA for the received funds to be recorded in Workday. Study teams may concurrently create an invoice in OnCore with the line items accounted for in the received funds but should wait to reconcile against it until the payment application process is completed in Workday as payment information will automatically populate in the Invoices and Receipts tab in Financials Console. When Workday accepts the invoice, the GCA Cash Team will match and apply the sponsor payment held on account in Workday and return a payment file to OnCore with payment information that can be used for reconciliation.
How will voided and amended invoices be handled for this integration?
For voided invoices:
They will display as (Void) next to the invoice number with a strikethrough of the dollar amount. As the integration is triggered and run overnight, it will inform Workday to update the status of the voided invoices. If payment was applied to an invoice, study teams would follow the current workflow of removing all applied funds to that invoice and voiding to cancel the invoice in OnCore. Once the integration is triggered and run, Workday will adjust the accounting accordingly and return the applied payment back to the study account. This returned fund will be held on account and can be used for other invoices.
For amended invoices:
A new invoice will be created in OnCore. After the invoice is finalized, it will be sent to Workday to be accepted. The old amended invoice will be crossed out in OnCore and adjusted accordingly in Workday.
The Summary Report the CTMS team will be monitoring will show that an invoice has been voided/amended.
OnCore has a report called “Unpaid Invoice Items Report” that shows invoices that do not have a payment applied to them. However, this report does not show whether funds have been received. Alternatively, another report called “CTMS Protocol Lifetime Receipts Report” shows funds received that have not been applied to an invoice in the “Unapplied Payments” column.
OnCore has a report called ” Subject Visit Tracking” that shows visit type (e.g., C2D1), visit status (e.g., occurred, planned, N/A), planned date, and visit date (i.e., the date the visit actually occurred) for the desired protocol and date range. Study teams can compare ACH payments against the visit status to verify whether visits have occurred. Another report called “Subject Visit Details” provides a similar view with the addition of the procedures that have been completed during the visit. Please reach out to your coordinator if visits have not occurred but are past the visit tolerance as both reports rely on timely and accurate subject visit tracking in OnCore.


