Project Governance
This project is governed by the following:
- Executive Sponsors—Comprised of leaders from Fred Hutch and UW, this group is accountable for providing strategic support across their organizations for the CTMS program, removing barriers noted by the team, and making key decisions raised by lead sponsors and program leadership.
- CTMS Program Office—This tri-institutional group is responsible for the ongoing operation of the CTMS. This Program Office is based at Fred Hutch and maintains neutrality across Fred Hutch and UW. It is led by a CTMS Program Director and supported by Institutional Leads (ILs) at each institution.
- CTMS Project Implementation Team (Sunset in 2021)—Led by a Project Director this team was organized into four workstreams – technical, functional, data migration & change management. Each workstream had leads, executors and subject matter experts.
- Extended Stakeholders—These groups include the CTMS/CTPI Steering Committee, Study Team Advisory Committee (STAC), The Research Charge Master Review Committee (RCRC), The Data Standards Committee (DSC), and the CTMS Access Review Committee (ARC). They are dedicated to provide specific advisory services related to the CTMS operations in their area of expertise.
Meet the Team
Executive Sponsors
T. Dellit
T. Lynch
CT Joint Oversight Committee
N. Davidson
Chair *
N. Robinson
FHCC
S. Neme
UW
K. Patrick
FHCC
P. Kruchek
Standing Guest
R. Mahan
UW
K. Stiffler
Standing Guest
* Chair to rotate annually
CT Joint Steering Committee
Co-Chairs
K. Stiffler
Co-Chair
P. Kruchek
Co-Chair
UW Medicine
W. Mitsuyama
UW Medicine
J. Reid
UW Medicine
A. Anderson
UW Medicine
K. Nasenbeny
UW Medicine
S. Mooney
UW Medicine
B. DeLair
UW Medicine
S. Sakiyama-Elbert
UW Medicine
A. Moore
UW Medicine
FHCC
Chief Clinical Ops
FHCC
M. Kondo
FHCC
N. Olsen
FHCC
T. Purcell
FHCC
T. Deeb
FHCC
W. Law
FHCC
T. McDonnell
FHCC
G. Subramanian
FHCC
Investigator
E. Yu
Investigator
M. Weiss
Investigator
CTMS Office/Joint
M. Van Rheen
CTMS Office/Joint
K. Avril
CTMS Office/Joint
FHCC/UW Contracting
C. Naduvil
FH/UW Contracting
C. Rhodes
FH/UW Contracting
FHCC/UW IRB
M. Scott
FHCC/UW IRB
J. Malone
FHCC/UW IRB
Executive Ops Committee
Advisory Committees
E. Yu
Investigator Co-Chair
M. Weiss
Investigator Co-Chair
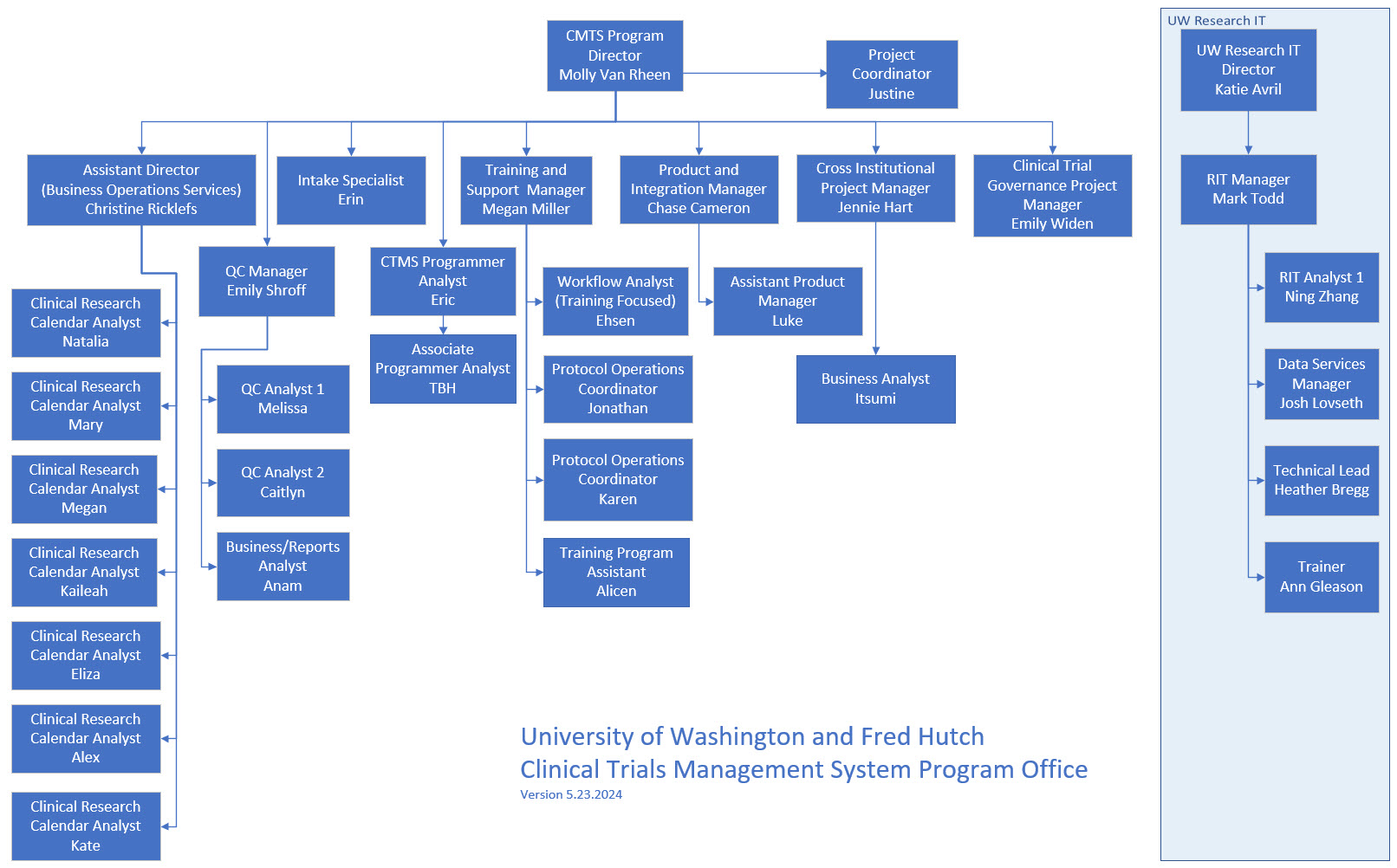
CTMS Program Office
Admin & General Program Operations
Molly Van Rheen
Director
Eric
Programmer Analyst
Erin
Protocol Operations Coordinator
Justine
Project Coordinator
Business Operations Services
Christine Ricklefs
Assistant Director
Alex
CTMS Calendar & Financial Analyst
Eliza
CTMS Calendar & Financial Analyst
Kaileah
CTMS Calendar & Financial Analyst
Kate
Lead CTMS Calendar & Financial Analyst
Mary
CTMS Calendar & Financial Analyst
Megan
CTMS Calendar & Financial Analyst
Natalia
CTMS Calendar & Financial Analyst
Product Management
Chase Cameron
Product & Integration Manager
Luke
IT Business Analyst
Quality Control & Reporting
Emily Shroff
QC Program Manager
Anam
Business & Reporting Analyst
Caitlyn
QC Analyst II
Melissa
QC Analyst I
Support Desk & Training
Megan Miller
Training Program Manager
Alicen
Training Specialist
Ehsen
Training and Support Specialist
Jonathan
Intake and Lead Support Specialist
Karen
Support Specialist
Cross-Institutional Project Management
Jennie Hart
Cross-Institutional Project Manager
Itsumi
Cross-Institutional Business Analyst
Clinical Trial Governance
Emily Widen
Clinical Trial Governance Project Manager
UW Research IT
Katie Avril
Director, Research Information Technologies
Mark Todd
Enterprise IT Research Manager
Josh
Lead Research IT Infrastructure Engineer
Ning
Sr. Software Engineer