10 Jan Ethical Dilemmas in Research: Insights from the ITHS Bioethics Team
The ITHS interview series is meant to shine a deserving spotlight on individuals who are doing critical but sometimes invisible work across the vast spectrum of translational science. In this installment, we sat down for a conversation with the expert consultants who make up the ITHS Bioethics Team to learn how they help researchers during study development. Expert consultation from this specialized team, based at Seattle Children’s, helps investigators consider perceived ethical issues that are anticipated to be complex i.e., when is a placebo-controlled trial justified. Members of the Bioethics Team, Benjamin Wilfond MD, Kate MacDuffie, PhD, and project coordinator Devan Duenas, sat down with the ITHS communications team for an interview about their new book, Challenging Cases in Research Ethics. It comprises decades of bioethics research published in the American Journal of Bioethics and is designed to be a resource for researchers, and students in healthcare research fields.
Can you start us out by helping us understand more about the fundamentals of bioethics?
Wilfond: I’ll give this a shot, as it’s something I’ve been working on explaining for the last 40 years. Bioethics is about helping people who face challenging decisions in healthcare or research where the best course of action isn’t always clear. In such cases, our goal is to collaboratively find a solution that is practical and acceptable to everyone involved. This is especially relevant in research, where researchers often face dilemmas where a solution seems ambiguous, and they are unsure where to seek help. A part of what we do is help them arrive at a solution themselves prior to grant or IRB submission.
Researchers often face dilemmas where a solution seems ambiguous, and they are unsure where to seek help. A part of what we do is help them arrive at a solution themselves.
Can you tell us a little bit more about the cases you profile in this book?
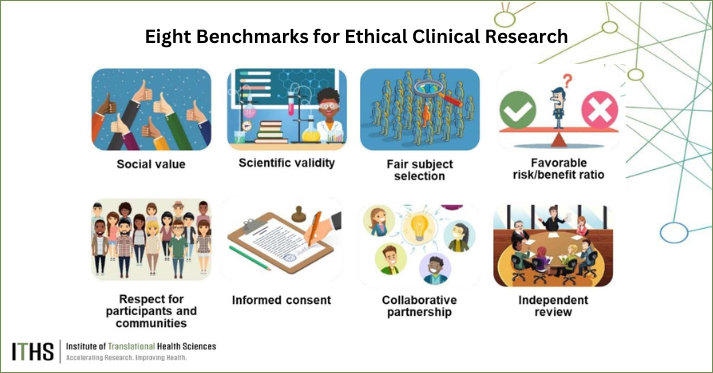
Duenas: The book compiles around 30 case studies originally published in the American Journal of Bioethics as part of the Challenging Cases in Research Ethics. These cases span diverse topics—like pediatrics, genetics, and consent issues, each featuring commentaries by several authors to showcase various ethical perspectives. Organized to serve as a teaching resource, the cases are grouped by thematic categories and structured around several key ethical principles in research, including scientific validity, fair subject selection, risk-benefit analysis, and informed consent. This framework, developed by Ezekiel Emanuel and Christine Grady over 20 years ago, helps readers explore the ethical dimensions of each case and encourages them to consider multiple principles when arriving at a solution. One unique feature of the book is its emphasis on the complexity of bioethics where different readers may reach varying conclusions based on the principles they prioritize. Rather than defining a single “right” answer, the book highlights the complex decision-making process involved in ethical problem-solving.
What inspired your team to write this book?
Wilfond: Inspiration to build out a comprehensive tool for bioethics stemmed from the team’s desire to make these ethical scenarios more accessible to educators and students. We had been publishing these cases online for about 10 years, but they were scattered and required people to search for them individually. At some point, while discussing ways to make these cases more accessible, someone suggested compiling them into a book. Our aim was to create a resource for those teaching research ethics. For instance, Katie Porter, who teaches research ethics at the University of Washington, is now using this book in her class. Ultimately, we wanted to create an easily accessible tool to help facilitate meaningful discussions on complex ethical research issues.
Can you share an interesting case featured in the book?
Wilfond: One of my all-time favorites is a case we’ve used for years to illustrate ethical principles in teaching. It involves researchers who conducted clinical trials in the early 1980s on antiviral drugs for newborns with congenitally-acquired herpes. These drugs proved effective, and today they’re commonly used. However, the researchers had questions about the long-term implications: would these individuals, as adults, remain contagious? They tried to follow up with the original study participants, now young adults, but encountered resistance because the parents, who had consented for their children, didn’t want to disclose the herpes diagnosis to their now-adult children. This raised ethical questions about whether researchers could contact the now-adults directly without parental involvement. It’s an interesting case because it explores competing rights to information and privacy, and how to balance them. This case magnifies the complexity of balancing privacy and right to information and is a great example of ethical decision making in healthcare research.
What do you want people to know about bioethics and your team’s role within ITHS?
MacDuffie: Some people combine bioethics with IRB (Institutional Review Board) or research regulations, assuming it’s just about saying “no” or pointing out what’s wrong. That’s a misconception. At ITHS, our team provides guidance to researchers, research staff, and research participants who have questions about any aspects of research study. We’re deeply committed to supporting research and ensuring it succeeds for everyone involved: researchers, participants, and society. We approach complex situations collaboratively, aiming to clear hurdles, not create them. We also bring a broad skill set to each project, constantly adapting as we dive into new areas. Many research teams find our involvement helpful that they consult us repeatedly and even bring us on as collaborators on their grants. “Collaboration” might be overused, but for us, it’s the perfect descriptor for what we do. We are genuinely committed to the success of research, research participants, and the society as a whole.
We are genuinely committed to the success of research, research participants, and the society as a whole.
How would you like people to view your team and what you offer?
Wilfond: Kate summarized it well earlier, so I’ll just add an illustration from one of the cases in the book about a longitudinal study called the “Dog Aging Project” at the University of Washington. The study investigates the aging process in dogs and explores a drug’s potential effects on longevity. Initially, an NIH reviewer criticized the study, questioning the ethics of extending a dog’s lifespan. The research team consulted us, and we helped them frame a response that aligned with established human aging research principles. The grant was funded, and as the study progressed, we continued collaborating with the team on various issues, from authorship to participant results. It’s an example of how the ITHS Bioethics Consultants work with teams not only to resolve immediate issues but to navigate ethical challenges as they arise. Essentially, we’re here to help whether it’s addressing a specific ethical issue or working as an ongoing collaborator.
Click here to learn more about the ITHS Bioethics Team and the services they offer!