Diversity in Clinical Trials Initiative
A partnership between the Office of Healthcare Equity, the Human Subjects Division, UW Medicine, UW School of Medicine, ITHS, and our community
Page last updated: Nov 12, 2024
The University of Washington’s Diversity in Clinical Trials Initiative (DCTI) aims to enhance representation of historically underrepresented communities among participants in clinical studies, ensuring that medical treatments and interventions are effectively studied across diverse populations, leading to more equitable healthcare outcomes for all individuals and better medicine.
The DCTI honors Washington Legislature House Bill 1745, while taking guidance from the FDA regarding the equitable selection of clinical trial participants and development of a diversity plan.
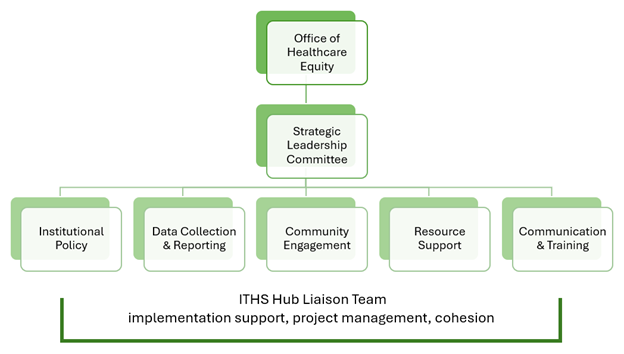
The University of Washington is currently reviewing and revising its clinical trial policies with deeper and more comprehensive diversity criteria to usher in a new era of inclusive medical research.
This page will serve as a comprehensive resource hub, providing information, updates, and resources related to the DCTI and its implementation efforts.
Implementation of these policies will be gradual and phased in throughout the next year and will apply to new studies only. Existing clinical trials will not be subject to the new requirements. The effective date for compliance will be one year from publication of the new policies. Please see the timeline below for more details.
DCTI Policy and Associated Guidance
Thank you to everyone who provided feedback on the proposed new SUPPLEMENT Diversity Plan for Clinical Trials and associated policy/guidance. The public comment period is now closed. HSD is busy evaluating all the comments and questions received.
The HSD intends to publish the final SUPPLEMENT and guidance this January 2025. Compliance with the new requirements would be one year from the publication date (i.e., January 2026) though preparatory work at the pre-award/study design stage will need to occur before then. The new requirements would only apply to new studies submitted to the IRB on or after the 2026 effective date.