The ITHS Research Coordinator Networking to Enhance Development (NED) Conference is taking place on January 30th. This is a free, annual, day‐long professional development conference for research coordinators held in Seattle. Participants attend both general sessions and choose relevant breakout sessions to expand their knowledge and skills. A catered lunch and collaborative activities offer coordinators a chance to develop contacts, share ideas, and learn from peers.
The theme for NED 2020 is Study Coordinator Leadership: From Theory to Action.
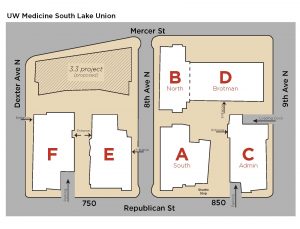
Attendees should plan to start the day at 8:00am at the UW South Lake Union C-Building for breakfast and check-in. Please see the bottom of this page for directions, parking information and a map of the SLU campus.